SCIENCE
GENE THERAPY PD
-
 Proprietary High-yield Cell-Line
Proprietary High-yield Cell-Line -
 Scalable, Robust and High-Yield Process
Scalable, Robust and High-Yield Process -
 Semi- and Automatic Filling Capacity
Semi- and Automatic Filling Capacity -
 Well-Controlled Cost of Goods
Well-Controlled Cost of Goods -
 Validated Methods for Product Release and Stability Program
Validated Methods for Product Release and Stability Program -
 In-Process Control for Consistent Product Quality
In-Process Control for Consistent Product Quality -
 Global Regulatory Compliance
Global Regulatory Compliance -
 Shorter Timeline for
Shorter Timeline for
GMP Readiness
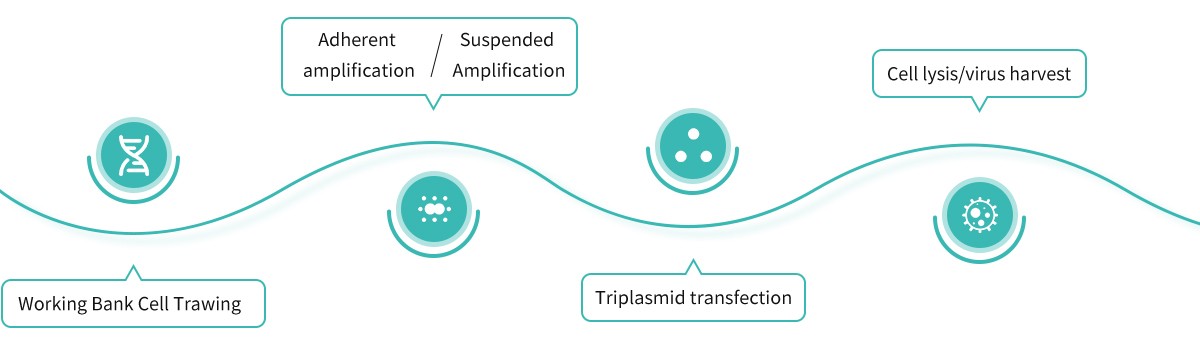
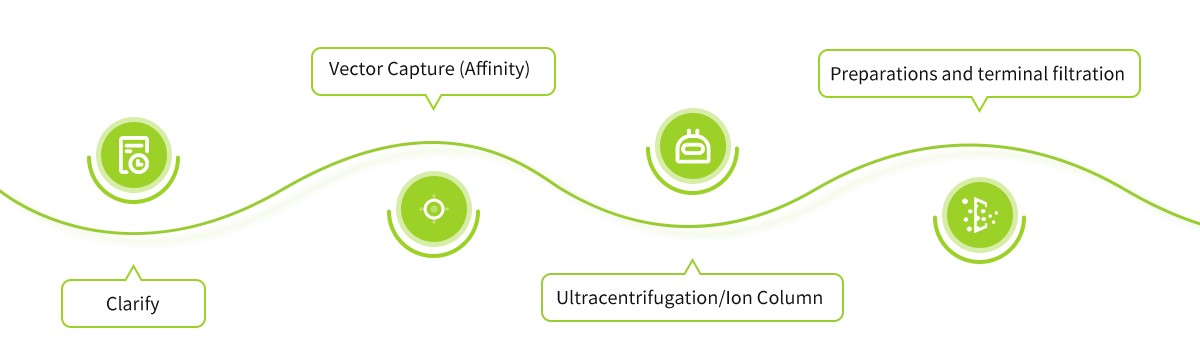
Robust & Flexible Process Development & Pilot Manufacturing Platforms in Wuhan
High-yield, Robust & Flexible Process Combination of Up- & Down-streams
- Upstream PD
- Downstream PD
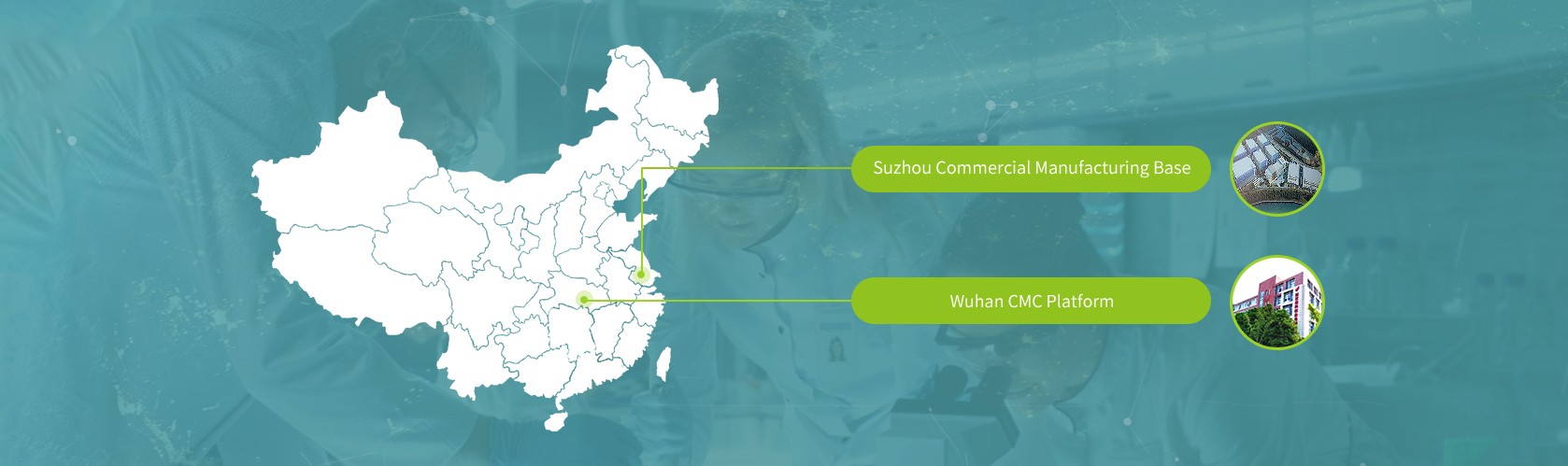
Suzhou Commercial Manufacturing Base
8,000 square meters of independent manufacturing base was completed
Designed by world-class designing companies according to international (NMPA, FDA, EMA) GMP standards
Flexible upstream (adherence/suspension), downstream and filling process combination to meet requirements of products with different characteristics
GMP cell bank preparation workshop, technology transfer laboratory and QC laboratory ensure rapid technology transfer and product release
Suffice the global commercial demand for the company’s all ophthalmic gene therapy products
Reserved the production capacity for external cooperation and the land for future commercial facilities

